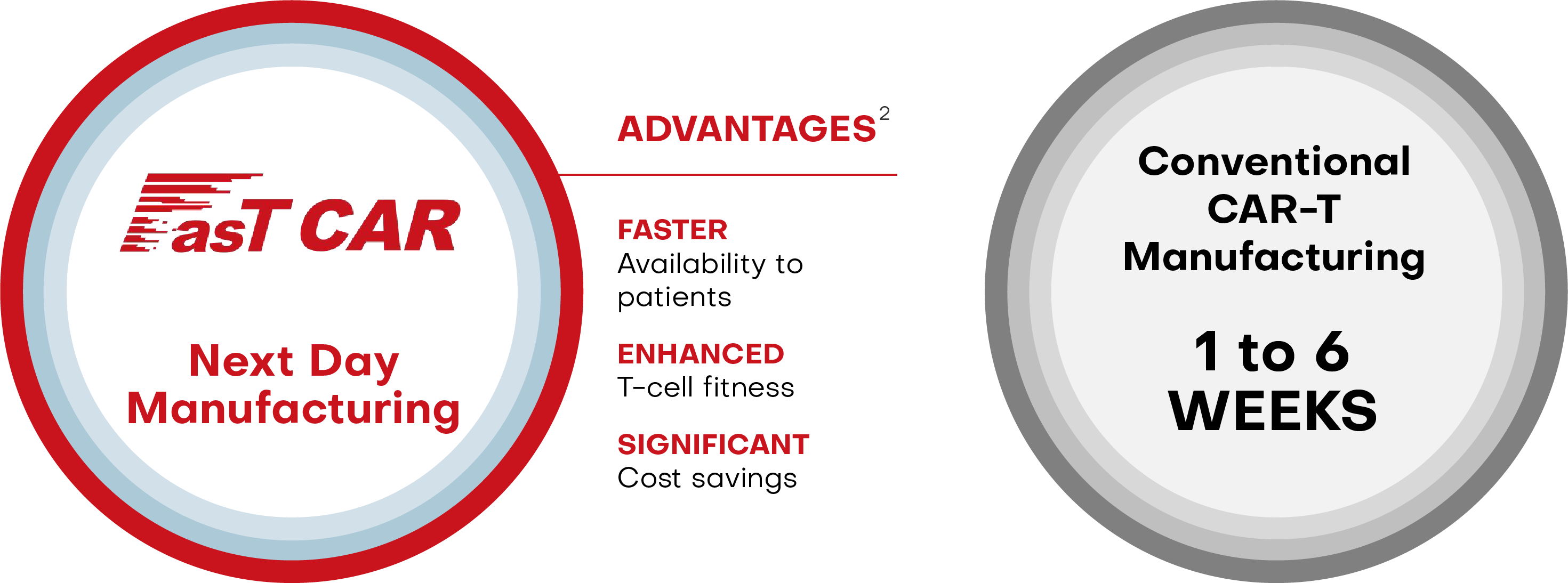
Introduced in 2017, FasTCAR is Gracell's rapid autologous CAR-T cell manufacturing platform, designed to pioneer next generation cell therapy for cancer and autoimmune diseases. FasTCAR shortens cell production from weeks to overnight, potentially reducing patient wait times to bring therapies to patients faster. Furthermore, our research shows that FasTCAR T-cells appear younger and 'less exhausted' than traditional CAR-T cells, making them more proliferative and effective at killing cancer cells1.
Our FasTCAR technology utilizes a fully-closed system manufacturing design, for improved production quality and operational consistency and reduced risk of contamination.
In 2022 and 2023, FasTCAR was named the winner of the Biotech Innovation category of the 2022 Fierce Life Sciences Innovation Awards and the Overall Immunology Solution of 2023 by BioTech Breakthrough Awards, for its ability to address major industry obstacles.
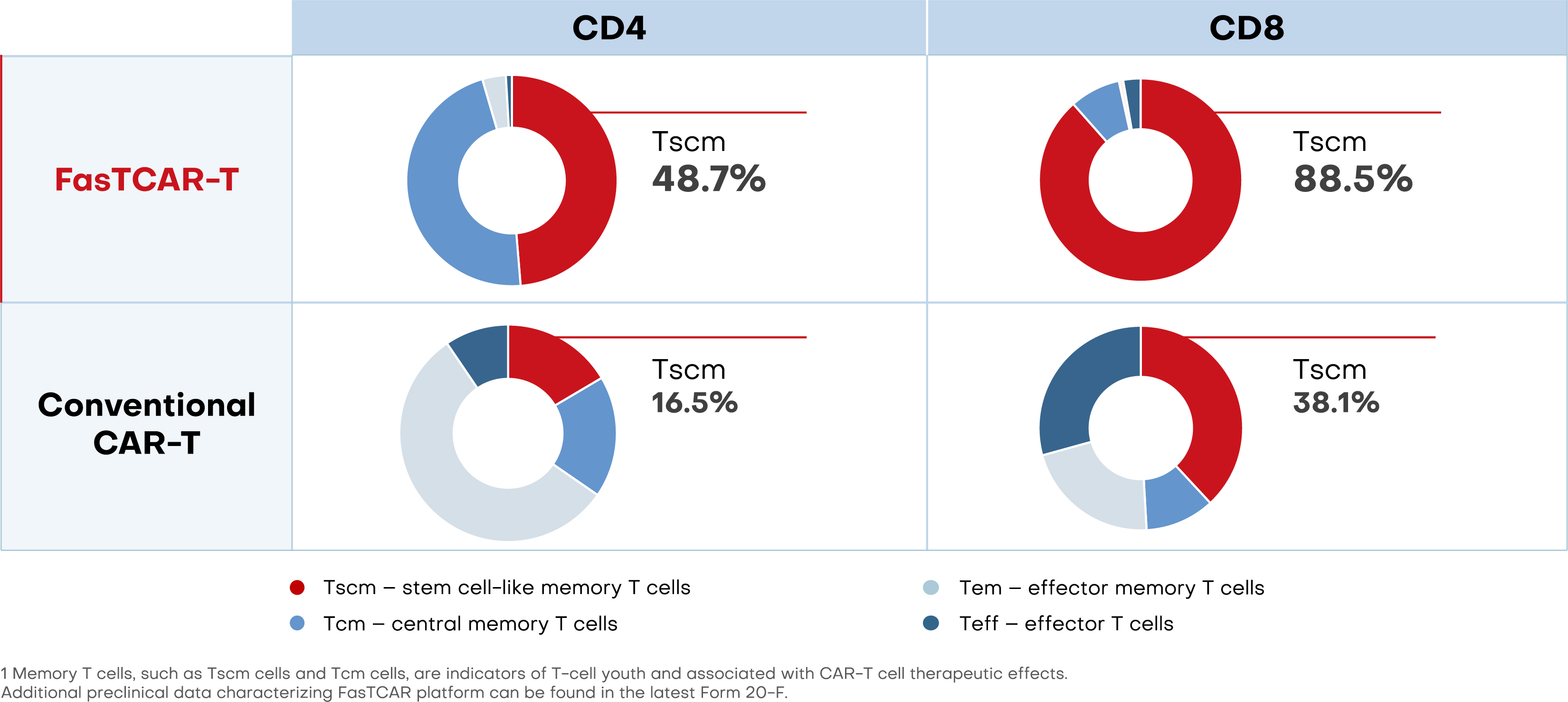
FasTCAR preserves high percentage of young Tscm cells1
With enhanced stemness and proliferation, FasTCAR-T cells may lead to lower dosage requirements and improved therapeutic results
The Science Behind FasTCAR:
FasTCAR vs Conventional CAR-T Manufacturing Process
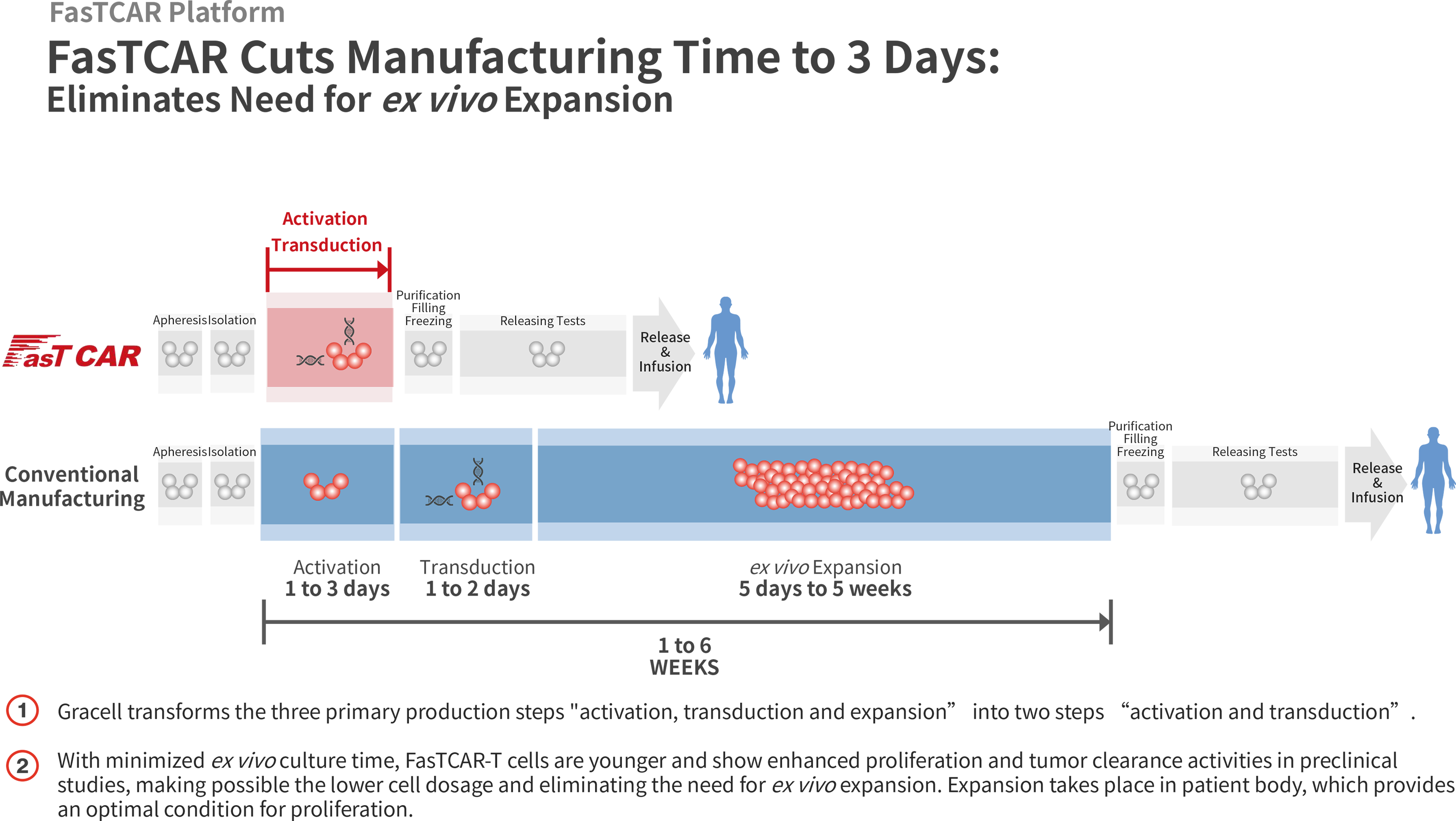
In the conventional CAR-T manufacturing process, a patient’s T cells are first activated using CD3 and/or CD28 antibodies, and then transduced by virus vectors to express one or more CARs. These engineered CAR-T cells are then expanded ex vivo before they are administered back into the human body. This process typically takes one to six weeks.
Using our proprietary FasTCAR platform, we are able to concurrently activate and transduce resting T cells into a single “concurrent activation-transduction” step using XLenti vectors derived from lentivirus that are of high quality and exhibit high gene transduction efficiency. As a result, after transduction, one or more CARs are integrated in the T cell genome and expressed stably. Based on our preclinical studies, these transduced T cells are highly active in proliferation and tumor cell clearance, and therefore can be administered to patients without the need for ex vivo cell expansion. With these innovations, our FasTCAR technology transforms the activation, transduction and expansion steps into a single “concurrent activation-transduction” step, as depicted in the figure below, significantly reducing the autologous CAR-T cell manufacturing time from an industry standard of one to six weeks and achieving next-day manufacturing.
We believe that our FasTCAR technology can be applied broadly to any CAR-T antigens and a variety of tumor markers, based on our clinical and preclinical studies. We are currently developing several autologous product candidates targeting hematologic malignancies, including RRMM, NDMM, B-NHL, SLE and AML 3.
2 Novel CD19 chimeric antigen receptor T cells manufactured next-day for acute lymphoblastic leukemia | Blood Cancer Journal; Next-day manufacture of a novel anti-CD19 CAR-T therapy for B-cell acute lymphoblastic leukemia: first-in-human clinical study - PubMed
3 RRMM: relapsed or refractory multiple myeloma; NDMM: newly-diagnosed multiple myeloma; B-NHL: B cell non-Hodgkin’s lymphoma; SLE: systemic lupus erythematosus; AML: acute myeloid leukemia
Fully-Closed Production Lines
Our Suzhou manufacturing facility established fully-closed production capabilities, designed to produce FasTCAR product candidates while reducing contamination risks and optimizing cost-efficiency. We believe these advantages, coupled with our ability to achieve next-day manufacturing for autologous CAR-T cells, allow us to improve productivity and scale up our production in a cost-efficient manner. Beyond China, we have also expanded our manufacturing capabilities to the United States to enable a local supply of high-quality novel cell therapies, including through collaborations with contract development and manufacturing organizations in the U.S.